Kisqali® Breast Cancer Drug Financial Assistance Program
Program Introduction
Breast cancer patients usually rely on medications to treat cancer. In view of the fact that the cost of medication has led to a significant financial burden on some patients, the Breast Cancer Drug Financial Assistance Program, established by Hong Kong Breast Cancer Foundation (HKBCF) and pharmaceutical companies, is dedicated to helping financially challenged breast cancer patients gain access to medications. In the hope that patients will keep a positive attitude in fighting against cancer and live a life.
1. About the Breast Cancer Drug Financial Assistance Program – KISQALI®
- Starting from Cycle 19, the patient can receive KISQALI®(ribociclib) for free from private clinics or private hospitals enrolled in the Program, until the regimen is changed by the doctor*.
- Registered patients will be paying at a similar price for KISQALI® as those attending public hospitals, regardless of where the patient is attending (private clinics or private hospitals).
- The application procedures are simple and the registered patient can collect the medication from their enrolled private doctor. The whole process does not involve a third party in examining the patient’s condition or receiving the medication.
* KISQALI® Treatment Cycle 1 to 18 will be at the patient’s own cost
2. Who is eligible for the Breast Cancer Drug Financial Assistance Program – KISQALI®?
Qualified applicants must fulfill ALL the criteria below:
- The applicant is not enrolled in other drug financial assistance programs simultaneously, namely the Community Care Fund and St. James’ Settlement.
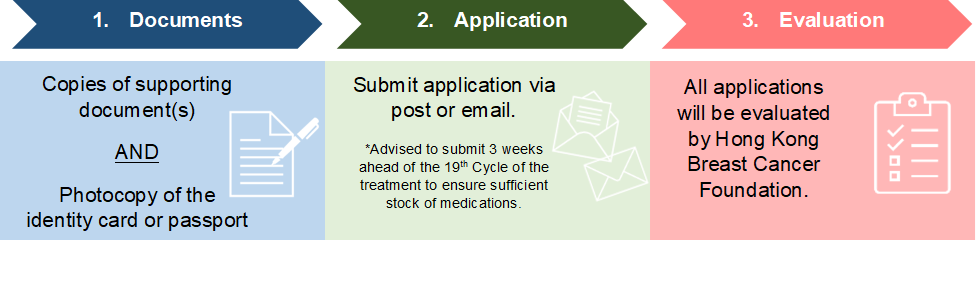
- It is advised to submit 3 weeks ahead of the 19th Cycle of the treatment to ensure sufficient stock of medications.
- The applicant should be receiving KISQALI® from private hospitals or private clinics and will be reaching Cycle 19.
- The first cycle of treatment should be initiated between 1 Jan 2021 and 14 Jan 2023.
3. Documents required for application
Applicants should submit the documents below for evaluation:
|
First-time application |
|
Second or later applications |
||
|
1 |
Original copy of a completed “Drug Subsidy Program – KISQALI® (ribociclib) Registration Form” with the signature of physician-in-charge enrolled in the Program |
|
1 |
To be provided by physician-in-charge Photocopy of Program Card OR Photocopy of Cycle Table A/B |
|
2 |
To be provided by physician-in-charge Photocopy of a completed Program Card with the signature of physician-in-charge enrolled in the Program OR Photocopy of Cycle Table A/B with the signature of physician-in-charge |
|
2 |
Photocopy of applicant’s HKID card or passport |
|
3 |
Photocopy of applicant’s HKID card or passport |
|
||
4. Application procedures
Please send the above listed documents, either by (i) email to bcsc-ap@hkbcf.org (Please put in the subject line: Application for Breast Cancer Drug Financial Assistance Program – Ribociclib”), or (ii) post to
Hong Kong Breast Cancer Foundation
22/F, Jupiter Tower, 9 Jupiter Street, North Point, Hong Kong
(Please mark on the envelope “Application for Breast Cancer Drug Financial Assistance Program – Ribociclib”.)
All applications will be evaluated by Hong Kong Breast Cancer Foundation.
Should you have any enquiries, please dial (852) 2525 6033.
5. Important notice
- All personal information collected will be treated in strictest confidence and in accordance to the Personal Data (Privacy) Ordinance.
- Hong Kong Breast Cancer Foundation reserves the right to make the final decision and to decline any application without providing reason.
- Hong Kong Breast Cancer Foundation may request further information and supporting documents from applicant, contact applicant’s physician-in-charge for more information and drug sponsors can change or stop the sponsorship at any time for any reason.
Novartis
Novartis is reimagining medicine to improve and extend people’s lives. As a leading multinational pharmaceutical company equipped with strong R&D capability, the company is committed to creating transformative treatments in areas of great medical need. Collaborating with a charitable organization, the company aims to provide financial support to breast cancer patients in need.
What is KISQALI®?
KISQALI® is an oral tablet that contains an active ingredient called ribociclib. It is the only CDK4/6 inhibitor that has proven to be effective in prolonging overall survival in premenopausal and postmenopausal women in clinical trials. It can inhibit the growth and differentiation of cancer cells. 1
KISQALI® can be used in hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer. Patients should be prescribed a combination therapy together with hormonal treatment. 1 Please consult your physician for more details.
How to adminster KISQALI®?
The usual course of treatment is 21 days KISQALI® followed by a 7-day break to complete a 28-day treatment course. 1 It is recommended to take the tablets at the same time each day. The tablets should be swallowed whole. Do not chew or crush the tablet. Avoid eating grapefruit and avoid drinking grapefruit juice. 1 The Drug Subsidy Program will be providing drug quantity based on the 21-day consumption.
Is KISQALI® effective?
International phase III clinical trials have demonstrated that KISQALI® together with endocrine therapy significantly bought to longer overall survival and progression-free survival to postmenopausal women when compared with those receiving hormonal treatment alone. 2,3 The latest clinical trial data in 2020 have shown that KISQALI® combination therapy delayed chemotherapy of 4 years (median) and prolonged overall survival of nearly 5 years (median). 4,5 These patients also reflected an improved quality of life. 6
What are the side effects of KISQALI®?
KISQALI® is generally well tolerated. A few patients experienced low white blood cell counts, and nausea and vomiting. 1
References:
- KISQALI® Prescribing information. September 2019.
- Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465-2472.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541-1547.
- Tripathy D, Im SA, Colleoni M, et al. Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with HR+/HER2- advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Poster presented at: San Antonia Breast Cancer Virtual Symposium; December 8-12, 2020.
- Novartis Kisqali® demonstrates nearly five years median overall survival in metastatic breast cancer. 9 December 2020. Available at: https://www.novartis.com/news/media-releases/novartis-kisqali-demonstrates-nearly-five-years-median-overall-survival-metastatic-breast-cancer. Accessed on 18 March 2021.
- Harbeck N, Franke F, Villanueva-Vazquez R, et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: results from a phase III randomized clinical trial (MONALEESA-7). Ther Adv Med Oncol. 2020;12:1-8.

